Technical Paper Title: Hydrogen Fuel Cell
Authors: M. SUMA & D. GANGA BHAVANI, 1st BTech, EEE
College: Prakasam Engineering College, Kandukur
ABSTRACT
World is witnessing a worsening global warming situation as power generation is continuously being increased throughout the world using fossil fuels. Higher energy generation through fossil fuel imparts environmental degradation and is now a matter of concern globally. The world population is also expected to double by the middle of the 21st century and as a consequence economic development will also grow. As a result global demand for energy is expected to increase substantially by 2050 (by about two to three times).
There is an energy technology that can eliminate both air pollution and foreign oil imports a device that is quiet, compact, flexible, highly efficient and exceptionally clean. It’s called the FUEL CELL. This nonpolluting power source is unique in its potential applications: it can provide energy for sources as large as a utility power station and as small as a smoke detector. It is perhaps the most important anti-pollution technology in our history.
“Whereas the 19th Century was the century of the steam engine and the 20th Century was the century of the internal combustion engine, it is likely that the 21st Century will be the century of the fuel cell.”
What are fuel cells? How do they work? Why are they so important? What are the next steps for development of this crucial technology? This paper presents a brief description about fuel cells.
1. INTRODUCTION OF FUEL CELL
What Is A Fuel Cell?
“Fuel cell is an electrochemical device that continuously converts the chemical energy of externally supplied fuel and oxidant directly to electrical energy”.
The easiest way to understand fuel cells is to think of them as a cousin to the ordinary battery. Both produce electricity through electrochemical reactions. The difference lies in a fuel cell’s ability to constantly produce electricity as long as it has a source of fuel where a battery needs to be recharged. Consequently, since a fuel cell does not store energy internally, a fuel cell will not “run down” like a battery. Fuel cells directly convert the fuel into electricity where a battery has to replenish its electricity from an external source.
History of fuel cell
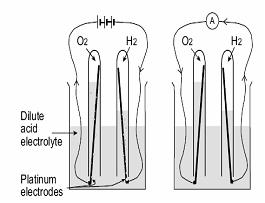
Sir William Grove (1811-96), a British lawyer and amateur scientist developed the first fuel cell in 1839. The principle was discovered by an accident during electrolysis experiment. When Sir William disconnected the battery from the electrolyzer and connected the two electrodes together, he observed a current flowing in the opposite direction, consuming the gases of hydrogen and oxygen. He called this device a “Gas Battery”. His gas battery consisted of platinum electrodes placed in test tubes of hydrogen and oxygen, immersed in a bath of dilute sulphuric acid. It generated voltages of about one volt.
There are many types of fuels for fuel cell: hydrogen, natural gas, methanol, petrol. In all cases, hydrogen is involved in the electrochemical reaction inside the fuel cell to generate electricity.
Why is hydrogen used as a fuel?
■ Hydrogen has the highest energy content per-unit-weight of any known fuel—52,000 Btu/lb (120.7 kJ/g).
■ Hydrogen burns cleanly. When hydrogen is burned with oxygen, the only by-products are heat and water. When it is burned with air, which is about 68 percent nitrogen, some oxides of nitrogen are formed.
Fuel cells operate on hydrogen or a variety of gaseous and liquid hydrocarbons. Electrolysis is used to produce hydrogen from water in areas where electricity is both abundant and available at a low cost. Fuel processing systems for reformation are used for hydrocarbon fuels, such as natural gas, methanol, ethanol, coal or gasoline. Hydrogen is produced by reforming hydrocarbon fuels at either a central fuel station dispensing hydrogen through distributed generation systems (off-board), or at a fuel cell location (on-board). While the use of fossil fuels to produce hydrogen does not promise zero emissions, reformation coupled with fuel cell technology can exploit existing fuel infrastructure, and will offer significant environmental improvements over traditional internal combustion engine systems. This is seen as an important step towards a hydrogen-driven economy. No greenhouse gas emissions exist with electrolysis using renewable sources of electricity, such as hydro, wind power, photovoltaics, geothermal or nuclear power.
2.Parts of a Fuel Cell:
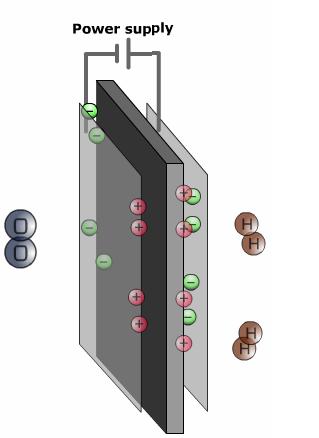
Anode: The anode, the negative side of the fuel cell, has several jobs. It conducts the electrons that are freed from the hydrogen molecules so that they can be used in an external circuit. Channels etched into the anode disperse the hydrogen gas equally over the surface of the catalyst.
Cathode: The cathode, the positive side of the fuel cell, also contains channels that distribute the oxygen to the surface of the catalyst. It conducts the electrons back from the external circuit to the catalyst, where they can recombine with the hydrogen ions and oxygen to form water.
Polymer electrolyte membrane: The polymer electrolyte membrane (PEM)—a specially treated material that looks something like ordinary kitchen plastic wrap—conducts only positively charged ions and blocks the electrons. The PEM is the key to the fuel cell technology; it must permit only the necessary ions to pass between the anode and cathode. Other substances passing through the electrolyte would disrupt the chemical reaction.
Catalyst
It accelerates the reactions at the electrodes?
3. Fuel cell operation

The fuel cell is an electrochemical devise, which converts chemical energy of the fuel to electricity by combining gaseous hydrogen with air in the absence of combustion. The basic principles of operation of the fuel cell is similar to that of the electrolyser in that the fuel cell is constructed with two electrodes with a conducted electrolyte between them. The heart of the cell is the proton conducting solid PEM. It is surrounded by two layers, diffusion and a reaction layer. Under constant supply of hydrogen and oxygen the hydrogen diffuses through the anode and the diffusion layer up to the platinum catalyst, the reaction layer. The reason for the diffusion current is the tendency of hydrogen oxygen reaction.
Two main electrochemical reactions occur in the fuel cell. One at the anode (anodic reaction) and one at the cathode.
At the anode, the reaction releases hydrogen ions and electrons whose transport is crucial to energy production.
H2->2H+ + 2e–
The hydrogen ion on its way to the cathode passes through the polymer membrane while the only possible way for the electrons is though an outer circuit. The hydrogen ions together with the electrons of the outer electric circuit and the oxygen which has diffused through the porous cathode reacts to water.
2H+ + ½ O2 + 2e– ->H2O
This process occurs in all types of fuel cells.
4. Types of Fuel Cells:
Fuel cells are classified primarily by the kind of electrolyte they employ. This determines the kind of chemical reactions that take place in the cell, the kind of catalysts required, the temperature range in which the cell operates, the fuel required, and other factors.
- Polymer Electrolyte Membrane (PEM) Fuel Cells
- Direct Methanol Fuel Cells
- Solid Oxide Fuel Cells
- Alkaline Fuel Cells
- Phosphoric Acid Fuel Cells
- Molten Carbonate Fuel Cells
- Regenerative Fuel Cells
- Comparison of Fuel Cell Technologies
In above only three technologies are most useful those are explained by below table
| PEMFC | SOFC | DMFC | |
| Electrolyte | Ion exchange membrane | ceramic | Polymer membrane |
| Operating temperature | 80 oc | 1,000 oc | 60-130 oc |
| Efficiency | 40-60% | 50-65% | 40% |
| Typical electrical
power |
Up to 250KW | >200KW | <10KW |
| Possible
Applications |
Vehicles
Small stationary |
Power stations | Portable
applications |
| Reactions
At anode: At cathode: |
2H2->4H+ + 4e–
4H+ +O2+ 4e–->H2O |
2H2+2o2--> + H2O+4e–
O2 + 4e– ->2o2- |
CH3OH+H2O->6H++
6e–+CO2 6H+ + 6e– 1½ O2->3H2O |
5. FUEL FOR FUEL CELL
Since most fuel cells are powered by hydrogen, one major issue is in which way hydrogen will be generated.
5a) Hydrogen production:
Ideally this would be done by non-polluting and renewable methods, such as solar, wind or hydro power tidal, etc.
In principal, electrolysis is the reverse reaction of a fuel cell; electricity is added to split water into its constituent elements resulting in the production of hydrogen and oxygen.
There are two types of production of hydrogen those are alkaline, PEM electrolysis.
Alkaline electrolysis:

In this electricity is used to split water into oxygen and hydrogen. the electrolyte contains hydrogen and oxygen atoms and when current is applied these are split into ions due to the current the ions will attracted to each electrode at anode oxygen is form and at cathode hydrogen is obtained.
This electricity can be produced by renewable energy sources
![]()
PEM electrolysis:
In PEM electrolysis the electrolyte is a solid polymer exchange membrane. It is reversal process to the PEM fuel cell in this on the anode side of the membrane water is split into hydrogen and oxygen. Hydrogen is then split into hydrogen ion that migrates through membrane and electron that follows the applied current on the cathode side hydrogen ion and electrons reacts and create hydrogen
At anode:
At cathode:
5b) Hydrogen storage:
Hydrogen can be stored in the following ways:
- Compressed gas storage (pressure storage)
- Liquid storage
- Metal halide storage
- Methanol storage
Hydrogen is difficult to store compared to gasoline. Gasoline is a liquid while hydrogen is a gas .Hydrogen at normal pressure has a volume 3100 times higher than gasoline.
Different hydrogen storage methods are used to reduce the storage volume.
Pressure storage:
Pressure storage of hydrogen reduces the storage volume .The higher pressure the lower the volume. However increasing the pressure costs energy.
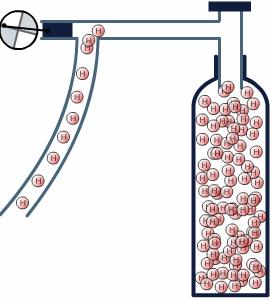
Today hydrogen can be stored under pressure up to 700 bars. The normal pressure for hydrogen storage is 200 bars. At 200 bars, hydrogen has a volume 13 times greater than gasoline, and at 700 bar 6, 4 times greater.
Liquid storage:

Hydrogen stored in liquid form reduces the storage volume. Cooling hydrogen to -253degrees makes it liquid. Cooling however costs energy and hydrogen in liquid form will diffuse out of the tank over time. Hydrogen in liquid form has a volume 3, 6 times higher has gasoline
Metal hydride storage:
Hydrogen can also be stored in metal powder; the so-called metal hydrides. When cooling is applied hydrogen atoms will move inside metal structures. To release hydrogen again heat must be applied.
Metal hydride storage is very safe due to a very low pressure and that very little hydrogen is in free form inside the tank.
Metal hydride holds a potential for storing hydrogen at very low volumes.
Methanol storage:
90% of the atoms in the universe are hydrogen, and many materials therefore contain hydrogen.
Methanol also contains hydrogen. Methanol is liquid and very similar gasoline. Hydrogen stored in methanol has a volume 1, 8 times higher than gasoline
6. Applications:
6.1) Transportation
Fuel cell technology promises to meet the most stringent emissions legislation. However, if fuel cells are to replace the internal combustion engine, the technology must not only meet tightening legislation, but also be able to reach operating temperature rapidly, provide competitive fuel economy and give a responsive performance. Proton exchange membrane fuel cells (PEMFC) are best placed to meet these requirements.
With a low operating temperature (80°C), PEMFC can reach operating temperature quickly. Able to respond rapidly to varying loads, this type is twice as efficient as internal combustion engines. PEMFC also have the highest power density from the current fuel cell range, a crucial factor when space maximization is such an important consideration in vehicle designs. Furthermore, the solid polymer electrolyte helps to minimize potential corrosion and safety management problems. In order to avoid catalyst poisoning at this low operating temperature PEMFC need uncontaminated hydrogen fuel. Most major vehicle manufacturers regard the PEMFC as the successor to the internal combustion engine. Successful tests of buses have already taken place in several cities and more are scheduled to follow, notably in Europe, where nine cities will trial three fuel cell buses from 2003.
6.2) Large Stationary
The most advanced fuel cells are presently large stationary units providing electricity and heat. Their attractiveness includes their efficiency and low emissions. They are also of use in areas not served by a national power grid or where the national grid is unreliable and backup power is required. With operating temperatures as low as 80°C, fuel cells can be installed in private households and light commercial operations as well as meeting all the energy requirements of large industrial operations.
So far fuel cell manufacturers have focused on non-residential applications. UTC Fuel Cells, for instance, has installed over 250 phosphoric acid fuel cells (PAFC) at a range of sites, including schools, office blocks and banking facilities. In the future, high temperature fuel cells, such as molten carbonate (MCFC) and solid oxide (SOFC), may be adapted for larger industrial applications. With operating temperatures between 600-1100°C these high temperature cells can tolerate a contaminated source of hydrogen and hence can use unreformed natural gas, diesel or gasoline. Furthermore, the heat generated can be used to produce electricity by driving steam turbines.
6.3) Small Stationary
There is also significant potential for small stationary units (which we have defined as anything with a power output below 10kW). In this field the heat and power requirements of private households or small businesses could be met by low temperature proton exchange membrane (PEM) or SOFC. Units could power individual houses or groups of homes and could be designed to meet all of the energy requirements of the inhabitants, or only the base load, with peak demands covered in another way.
House hold usage of fuel cell:
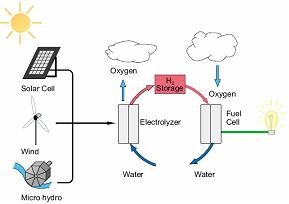
The renewable energy is not a stable energy source. Power is only produced when sun is shining and wind is blowing (i.e. when presence of alternative energy sources) there fore the excess of energy can be stored in the form hydrogen through electrolysis. And when the electricity is needed (usually night periods) the stored hydrogen can be transformed into electrical energy through fuel cell. The side figure shows the usage of fuel cell
6.4) Portable
Fuel cells promise to be an important source of power for mobile electronic devices, offering key advantages over conventional batteries, such as increased operating times, reduced weight and ease of recharging.
At present most research has focused on a variation of the low temperature proton exchange membrane (PEM) fuel cell, the direct methanol fuel cell (DMFC). As the name implies these fuel cells run on a methanol-water mix fed directly into the unit without prior reforming. Using methanol, DMFCs offer a great advantage over solid batteries in that recharging will just involve refilling with the liquid fuel.
6.5) Military
Military applications are expected to be a significant niche market for fuel cell technology. Their efficiency, versatility, extended running time and quiet operation make fuel cells extremely well suited for the power needs of military services.
In various forms, fuel cells could provide power for the majority of military equipment from portable handheld devices used in the field to land and sea transportation.
7. BENEFITS AND OBSTACLES TO THE SUCCESS OF FUEL CELLS
7.1) Benefits
• Fuel cells are efficient. Fuel cell system efficiency is independent of the rated power above 100 kW, unlike oil, gas or coal burning power plants, where the efficiency is constant only at the megawatt power level. Even at the 40% of the rated load, a fuel cell has almost the same efficiency as that of the full load.
• Fuel cells are clean. If hydrogen is the fuel, there are no pollutant emissions from a fuel cell itself, only the production of pure water. In contrast to an internal combustion engine, a fuel cell produces no emissions of sulphur dioxide, which can lead to acid rain, nor nitrogen oxides which produce smog nor dust particulates.
• Fuel cells are quiet. A fuel cell itself has no moving parts, although a fuel cell
system may have pumps and fans. As a result, electrical power is produced relatively silently. Many hotels and resorts in quiet locations, for example, could replace diesel engine generators with fuel cells for both main power supply or for backup power in the event of power outages.
• Fuel cells are modular. That is, fuel cells of varying sizes can be stacked together to meet a required power demand. As mentioned earlier, fuel cell systems can provide power over a large range, from a few watts to megawatts.
• Fuel cells are environmentally safe. They produce no hazardous waste products, and their only by-product is water (or water and carbon dioxide in the case of methanol cells). Fuel cells are also able to respond fast to load changes, because the electricity is generated by a chemical reaction.
7.2) Obstacles
At present there are many uncertainties to the success of fuel cells and the development of a hydrogen economy:
• Fuel cells must obtain mass-market acceptance to succeed. This acceptance
depends largely on price, reliability, longevity of fuel cells and the accessibility and cost of fuel. Compared to the price of present day alternatives e.g. diesel-engine generators and batteries, fuel cells are comparatively expensive. In order to be competitive, fuel cells need to be mass produced less expensive materials developed.
• An infrastructure for the mass-market availability of hydrogen, or methanol fuel initially, must also develop. At present there is no infrastructure in place for either of these fuels. As it is we must rely on the activities of the oil and gas companies tointroduce them. Unless motorists are able to obtain fuel conveniently and affordably,a mass market for motive applications will not develop.
• At present platinum is a key component to fuel cells. Platinum is a scarce natural resource; the largest supplies to the world platinum markets are from South Africa,Russia and Canada. Shortages of platinum are not anticipated, however changes inGovernment policies could affect the supply.
• Fuelling fuel cells is still a major problem since the production, transportation, distribution and storage of hydrogen is difficult.
• Fuel cells are in general slightly bigger than comparable batteries or engines. However, the size of the units is decreasing.
8. CONCLUSION
As our demand for electrical power grows, it becomes increasingly urgent to find newways of meeting it both responsibly and safely. In the past, the limiting factors of renewable energy have been the storage and transport of that energy. With the use of fuel cells and hydrogen technology, electrical power from renewable energy sources can be delivered where and when required, cleanly, efficiently and sustainably.
In the hydrogen economy… India will enjoy a secure, clean, and prosperous energy sector that will continue for generations to come. Indian consumers will have access to hydrogen energy to the same extent that they have access to gasoline, natural gas, and electricity today. It will be produced cleanly, with near-zero net carbon emissions, and it will be transported and used safely. It will be the ‘fuel of choice’ for Indian businesses and consumers.
India as a developed nation
Abdul kalam’s vision 2020 is not so far .2020 is the year to see India as a developed nation. For that we require surplus of energy and it is possible by using hydrogen energy in form of fuel cells since it is very abundant than any other fossil fuel